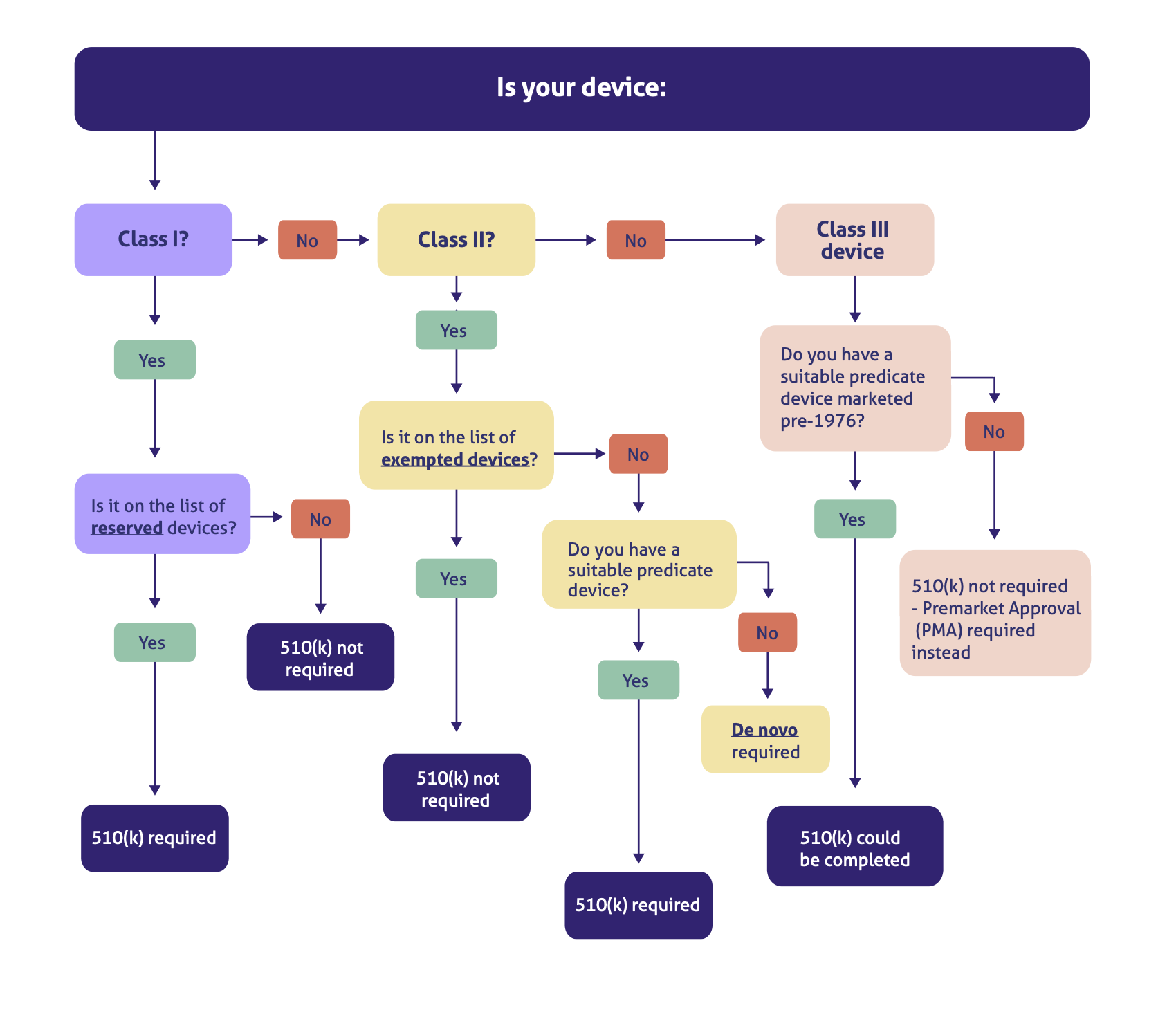
Pmda Medical Device Classification . development of drugs, medical devices, regenerative medicines and in vitro diagnostics. General medical devices (notification is required) class ii: In japan, medical devices are classified into four classes based on the risk level; 9 rows class i: in japan, medical devices are classified into four classes based on the risk level ; list of guidance documents: classification of medical devices. standards in regulation for medical devices. 2021/10/08 page last updated medical device nomenclaturemedical device nomenclature nomenclature definition:. [2024/08/15] <md>information about standards is updated. Controlled medical devices (designated products.
from www.qualio.com
[2024/08/15] <md>information about standards is updated. In japan, medical devices are classified into four classes based on the risk level; standards in regulation for medical devices. 9 rows class i: development of drugs, medical devices, regenerative medicines and in vitro diagnostics. in japan, medical devices are classified into four classes based on the risk level ; classification of medical devices. list of guidance documents: 2021/10/08 page last updated medical device nomenclaturemedical device nomenclature nomenclature definition:. Controlled medical devices (designated products.
The 3 FDA medical device classes differences and examples explained
Pmda Medical Device Classification in japan, medical devices are classified into four classes based on the risk level ; 2021/10/08 page last updated medical device nomenclaturemedical device nomenclature nomenclature definition:. classification of medical devices. in japan, medical devices are classified into four classes based on the risk level ; development of drugs, medical devices, regenerative medicines and in vitro diagnostics. standards in regulation for medical devices. In japan, medical devices are classified into four classes based on the risk level; [2024/08/15] <md>information about standards is updated. list of guidance documents: General medical devices (notification is required) class ii: 9 rows class i: Controlled medical devices (designated products.
From mindmachineco.com
Medical Device Classifications FDA vs EMA vs MDD vs PMDA Pmda Medical Device Classification in japan, medical devices are classified into four classes based on the risk level ; General medical devices (notification is required) class ii: Controlled medical devices (designated products. 9 rows class i: 2021/10/08 page last updated medical device nomenclaturemedical device nomenclature nomenclature definition:. list of guidance documents: classification of medical devices. standards in regulation. Pmda Medical Device Classification.
From mavink.com
Fda Medical Device Classification Chart Pmda Medical Device Classification in japan, medical devices are classified into four classes based on the risk level ; 9 rows class i: 2021/10/08 page last updated medical device nomenclaturemedical device nomenclature nomenclature definition:. General medical devices (notification is required) class ii: [2024/08/15] <md>information about standards is updated. development of drugs, medical devices, regenerative medicines and in vitro diagnostics. Controlled. Pmda Medical Device Classification.
From www.slideserve.com
PPT Classification of Medical Devices Clinical Evaluation and Conformity Assessment Pmda Medical Device Classification General medical devices (notification is required) class ii: [2024/08/15] <md>information about standards is updated. development of drugs, medical devices, regenerative medicines and in vitro diagnostics. standards in regulation for medical devices. in japan, medical devices are classified into four classes based on the risk level ; classification of medical devices. 9 rows class i: . Pmda Medical Device Classification.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Pmda Medical Device Classification development of drugs, medical devices, regenerative medicines and in vitro diagnostics. 2021/10/08 page last updated medical device nomenclaturemedical device nomenclature nomenclature definition:. General medical devices (notification is required) class ii: Controlled medical devices (designated products. In japan, medical devices are classified into four classes based on the risk level; in japan, medical devices are classified into four. Pmda Medical Device Classification.
From coastbiomed.com
UNDERSTANDING MEDICAL EQUIPMENT CLASSIFICATION Coast Biomedical Equipment Pmda Medical Device Classification standards in regulation for medical devices. Controlled medical devices (designated products. 2021/10/08 page last updated medical device nomenclaturemedical device nomenclature nomenclature definition:. 9 rows class i: General medical devices (notification is required) class ii: in japan, medical devices are classified into four classes based on the risk level ; [2024/08/15] <md>information about standards is updated. In. Pmda Medical Device Classification.
From www.simplerqms.com
Medical Device Classification (FDA & EU MDR) SimplerQMS Pmda Medical Device Classification Controlled medical devices (designated products. development of drugs, medical devices, regenerative medicines and in vitro diagnostics. In japan, medical devices are classified into four classes based on the risk level; 9 rows class i: classification of medical devices. in japan, medical devices are classified into four classes based on the risk level ; 2021/10/08 page. Pmda Medical Device Classification.
From chinameddevice.com
CFDA New Medical Device Classification Catalog China Med Device Pmda Medical Device Classification [2024/08/15] <md>information about standards is updated. list of guidance documents: General medical devices (notification is required) class ii: standards in regulation for medical devices. in japan, medical devices are classified into four classes based on the risk level ; In japan, medical devices are classified into four classes based on the risk level; classification of medical. Pmda Medical Device Classification.
From odoman.com
The 3 FDA Medical Device Classes [Differences and Examples Explained] (2023) Pmda Medical Device Classification standards in regulation for medical devices. General medical devices (notification is required) class ii: 9 rows class i: development of drugs, medical devices, regenerative medicines and in vitro diagnostics. [2024/08/15] <md>information about standards is updated. classification of medical devices. in japan, medical devices are classified into four classes based on the risk level ; . Pmda Medical Device Classification.
From dxoobywxs.blob.core.windows.net
Diagnostic Medical Device Classification at Crouse blog Pmda Medical Device Classification 9 rows class i: in japan, medical devices are classified into four classes based on the risk level ; [2024/08/15] <md>information about standards is updated. General medical devices (notification is required) class ii: In japan, medical devices are classified into four classes based on the risk level; 2021/10/08 page last updated medical device nomenclaturemedical device nomenclature nomenclature. Pmda Medical Device Classification.
From www.vrogue.co
The 3 Fda Medical Device Classes Differences And Exam vrogue.co Pmda Medical Device Classification in japan, medical devices are classified into four classes based on the risk level ; General medical devices (notification is required) class ii: [2024/08/15] <md>information about standards is updated. In japan, medical devices are classified into four classes based on the risk level; list of guidance documents: classification of medical devices. development of drugs, medical devices,. Pmda Medical Device Classification.
From japanhpn.org
Japan Health Policy NOW 6.4 Medical Devices Pmda Medical Device Classification in japan, medical devices are classified into four classes based on the risk level ; standards in regulation for medical devices. development of drugs, medical devices, regenerative medicines and in vitro diagnostics. 2021/10/08 page last updated medical device nomenclaturemedical device nomenclature nomenclature definition:. General medical devices (notification is required) class ii: [2024/08/15] <md>information about standards is. Pmda Medical Device Classification.
From docs.oracle.com
Device Classification Pmda Medical Device Classification In japan, medical devices are classified into four classes based on the risk level; standards in regulation for medical devices. development of drugs, medical devices, regenerative medicines and in vitro diagnostics. 2021/10/08 page last updated medical device nomenclaturemedical device nomenclature nomenclature definition:. in japan, medical devices are classified into four classes based on the risk level. Pmda Medical Device Classification.
From www.vrogue.co
Classification Of Medical Devices vrogue.co Pmda Medical Device Classification Controlled medical devices (designated products. [2024/08/15] <md>information about standards is updated. 2021/10/08 page last updated medical device nomenclaturemedical device nomenclature nomenclature definition:. In japan, medical devices are classified into four classes based on the risk level; General medical devices (notification is required) class ii: development of drugs, medical devices, regenerative medicines and in vitro diagnostics. standards in. Pmda Medical Device Classification.
From mindmachineco.com
Medical Device Classifications FDA vs EMA vs MDD vs PMDA Pmda Medical Device Classification 2021/10/08 page last updated medical device nomenclaturemedical device nomenclature nomenclature definition:. Controlled medical devices (designated products. development of drugs, medical devices, regenerative medicines and in vitro diagnostics. in japan, medical devices are classified into four classes based on the risk level ; list of guidance documents: [2024/08/15] <md>information about standards is updated. General medical devices (notification. Pmda Medical Device Classification.
From mungfali.com
Classification Of Medical Devices Pmda Medical Device Classification standards in regulation for medical devices. development of drugs, medical devices, regenerative medicines and in vitro diagnostics. In japan, medical devices are classified into four classes based on the risk level; General medical devices (notification is required) class ii: classification of medical devices. 9 rows class i: in japan, medical devices are classified into four. Pmda Medical Device Classification.
From www.vrogue.co
The 3 Fda Medical Device Classes Differences And Exam vrogue.co Pmda Medical Device Classification classification of medical devices. development of drugs, medical devices, regenerative medicines and in vitro diagnostics. 2021/10/08 page last updated medical device nomenclaturemedical device nomenclature nomenclature definition:. [2024/08/15] <md>information about standards is updated. General medical devices (notification is required) class ii: standards in regulation for medical devices. In japan, medical devices are classified into four classes based. Pmda Medical Device Classification.
From meddev-info.blogspot.com
Medical Device Regulation Basics US FDA Medical Device Classification Pmda Medical Device Classification Controlled medical devices (designated products. General medical devices (notification is required) class ii: [2024/08/15] <md>information about standards is updated. standards in regulation for medical devices. in japan, medical devices are classified into four classes based on the risk level ; classification of medical devices. 2021/10/08 page last updated medical device nomenclaturemedical device nomenclature nomenclature definition:. . Pmda Medical Device Classification.
From dxoustrzl.blob.core.windows.net
Fda Medical Device Classification Examples at Daniel Auten blog Pmda Medical Device Classification [2024/08/15] <md>information about standards is updated. Controlled medical devices (designated products. classification of medical devices. standards in regulation for medical devices. development of drugs, medical devices, regenerative medicines and in vitro diagnostics. General medical devices (notification is required) class ii: in japan, medical devices are classified into four classes based on the risk level ; . Pmda Medical Device Classification.